How Many Electrons Are in Hydrogens Outer Shell
The second shell can hold 8. 8 How many valence electrons are in hydrogens outermost energy level.
Hydrogen Has How Many Outer Shell Electrons Socratic
6 How do you find the number of electrons in the outer shell.
. 9 How many total electrons are now in the outer energy level of the carbon atom. The maximum number of electrons you will find in any shell is 32. 2-8-8 As you learn about elements with more than eighteen electrons you will find that shell three can hold more than eight.
1 Show answers Another question on Chemistry. Each of the hydrogen atoms has their 1 electron 1 shared electron to fill their shell with 2 electrons. How many electrons are in the outer shell of a hydrogen atom.
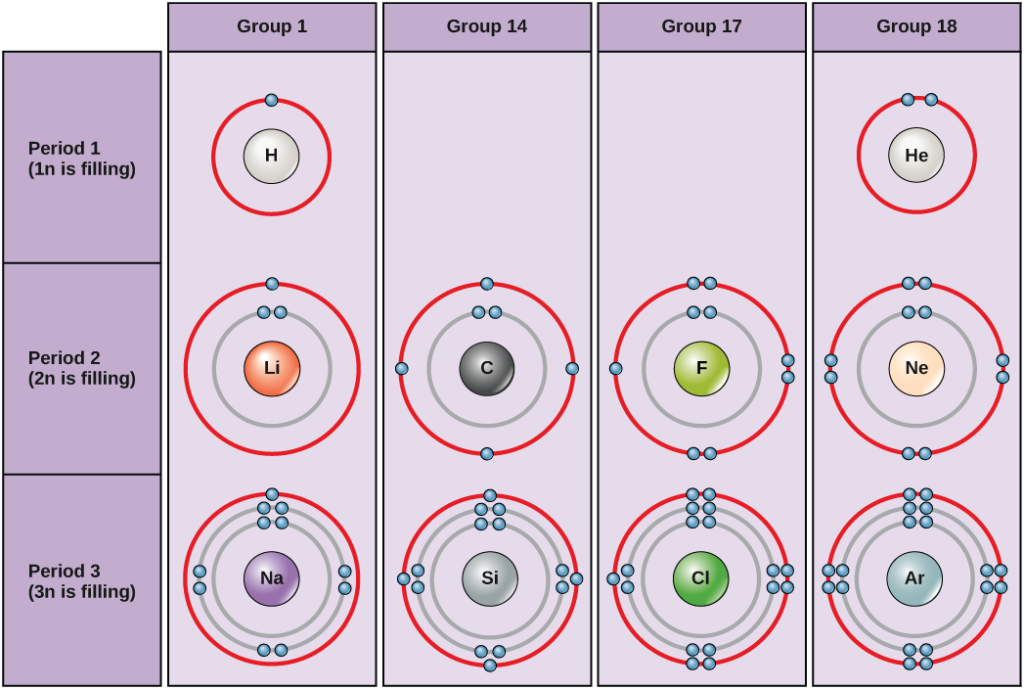
TLDR There is 1 electron in the outer shell of hydrogen. Hydrogen has 1 outer shell which contains 1 electron. As seen by the periodic table Hydrogen has 1 electron this is evident from the lower number on Hydrogen which tells you how many neutrons and electrons the element has.
14 Which is the outermost shell of hydrogen atom. A carbon atom can form covalent bonds with how many hydrogen atoms. 15 What is a full outer shell of electrons.
Tramwayniceix and 3 more users found this answer helpful. Carbon has 4 electrons and hydrogen has 1 electron in its outermost electron shell. Each shell can contain only a fixed number of electrons.
Carbon has six protons in its nucleus and six electrons orbiting around it. The M shell contains only eight electrons. 13 How many electrons are there in outermost shell.
One could even suggest the third shell is not the outermost. The shell is the same as the principle quantum number. The general formula is that the.
That means there is only one electron in a normal. Oxygen needs two electrons to fill its outermost electron shell. By bonding with two hydrogen atoms oxygen has its 6 outer shell electrons 2 shared electrons from the hydrogen making 8 and a filled shell makes a happy atom.
For example in the ground state of hydrogen there are no electrons in the third shell at all. 7 What is the total no of valence electron in h2o. 4 rows The first shell closest to the nucleus can hold two electrons.
Hydrogen has one electron to play with. 13 What element has 7 valence electrons and 2 shells. The first shell can hold up to two electrons the second shell can hold up to eight 2 6 electrons the third shell can hold up to 18 2 6 10 and so on.
The M shell can actually hold up to 18 electrons while moving to higher atomic numbers. How many electrons are in carbon. 10 How many more electrons will it take to fill the outermost energy level.
However if you mean how many are capable of being held in the third shell then the general formula is 2n². There is only one electron inhabiting hydrogen and the compound therefore only has one shell effectively making that shell the outer shell. Which of the following is not one of the steps in the scientific method a.
This makes carbon an element with a total of 14 electrons. How many electrons are found in their outer shell. For the element of HYDROGEN you already know that the atomic number tells you the number of electrons.
The K shell contains only two electrons. 12 What group is fluorine in. 11 How many valence electrons are in a fluorine atom and a fluoride ion quizlet.
Therefore an oxygen atom needs two hydrogens to bond to in order to complete its shell. Once a shell is full the next electron that is added must move to the next outer shell. If Hydrogen has 1 electron it can only have 1 outer shell or orbital as an electron cannot by split across shells.
See answer 1 Hydrogen has 1 electron in its outer shell and in fact only 1 electron in total. What kind of intermolecular forces act between a hydrogen fluoride molecule and. The L shell contains only eight electrons.
Chem4kids Com Hydrogen Orbitals And Compounds
No comments for "How Many Electrons Are in Hydrogens Outer Shell"
Post a Comment