Reduction of 4 Tert Butylcyclohexanone Mechanism
Stock GC-MS 4-tert-butylcyclohexanoneStock IR 4-tert-butylcyclohexanoneSample NMR 4-tert-butylcyclohexanol and 4-tert-butylcyclohexanone not available for submission for creditStock NMR 4-tert-butylcyclohexanone available for submission for credit see laboratory manual for detailsFrequently Asked Questions. Write the reaction mechanism and explain why the trans product predominates.
Solved The Mechanism For Oxidation And Reduction Of Chegg Com
It is clear that the trans product was formed in the sodium borohydride reduction of 4-tert-butylcyclohexanone.
. Ether is added to the separatory funnel. We did sodium borohydride reduction of 4-tert-butylcyclohexanone in lab recently. Sommerville and Theimer have.
After further stirring the mixture of liquids this mixture is transferred to a separatory funnel. Who are the experts. Science Chemistry QA Library Write a mechanism for the reduction of 4-tert-Butylcyclohexanone with NaBH4.
The liquid-phase reduction of 4- tert -butylcyclohexanone into 4- tert -butylcyclohexanol by reaction with isopropanol at 355 K has been studied on solid bases. The mechanism for oxidation and reduction of 4-tert-butylcyclohexanol and 4-tert-butylcyclohexanone respectively. Figure 3 Stereospecific Mechanism of Reduction of 4 tert butylcyclohexanone with from CHEM 2081 at Auburn University.
Mechanism of the reduction of 4-tert-butylcyclohexanone. The Meerwein-Pondorff-Verley MPV reaction using aluminum isopropoxide in the presence of isopropyl alcohol provides a reversible reduction of ketones and aldehydes3 In the MPV. In sharp contrast MPV reduction of 4-tert-butylcyclohexanone yielded 95 conversion in 4 days similar to those reported for Zr-TUD-1.
Mechanism for the reduction of 4-tert-butylcyclohexanone with sodium borohydride in. 4-tert-Butylcyclohexanone is dissolved in cold ethanol. Discussion The percent yield was around 76 percent so the reaction can be considered successful.
The trans product is the product that is most dominant in this situation because it is a more stable conformation and easier to take on. Using the starting material 4-t-butylcyclohexanone and the. 3 4 Eliel and Ro 5 obtained cis-rich 4-tert-butylcyclohexanol by the reduction of 4-tert-butylcyclohexanone with hydrogen on platinum oxide in glacial acetic acid containing some hydrogen chloride.
In the experiment 4 the reduction reaction is what is being performed. Show electron flow for ALL steps. The thermodynamically favored product was formed because sodium borohydride is not a sterically hindered reducing agent and is therefore able to attack the 4- tert -butylcyclohexanone from the top face of the molecule despite the presence of the large.
The mechanism involved Lewis work Al species of strong Lewis acidity and catalyzed the reduction acid sites described as Al species partially attached to the of cinnamaldehyde into isopropyl-cinnamyl ether thus suggesting lattice 8. Significantly less basic and the acid-base reaction is much slower. After reduction and before liquid-liquid extraction we added distilled water and 3M HCl to the solution until H gas ceased production.
Reduction of 4-tert-butylcyclohexanone - CM2191SUBSCRIBEIf you would like to have more chemistry fun and learn about cool science subscribe to this chan. This mixture is stirred and sodium hydroxide is added. NaBEA zeolites retained extraframe- 4-tert-butylcyclohexanol.
Discover the worlds research 20 million members. Experts are tested by Chegg as specialists in their subject area. Sodium Borohydride Reduction of 4-t-Butylcyclohexanone.
4 unhindered 10. We review their content and use your feedback to keep the quality high. Stereoselective Reduction of 4-tert-Butylcyclohexanone.
Mixed oxides obtained by calcination of hydrotalcites NaBEA zeolites KFalumina and La 2 O 3 characterized by calorimetric FTIR adsorption of CO 2 and FTIR pyridine adsorption. The mechanism of the reduction of 4-tert-butylcyclohexanone is shown below. Be as complete as possible and include all relevant resonance structures.
Bu 3 very hindered. The miniscale procedure can be found of page 240-243. Chemistry questions and answers.
Mechanism and as a result have quite different selectivities which are often complementary to the hydride reagents discussed. 4-tert-Butylcyclohexanol has been prepared from p-tert-butylphenol by reduction under a variety of conditions. Bu 3 more hindered 10.
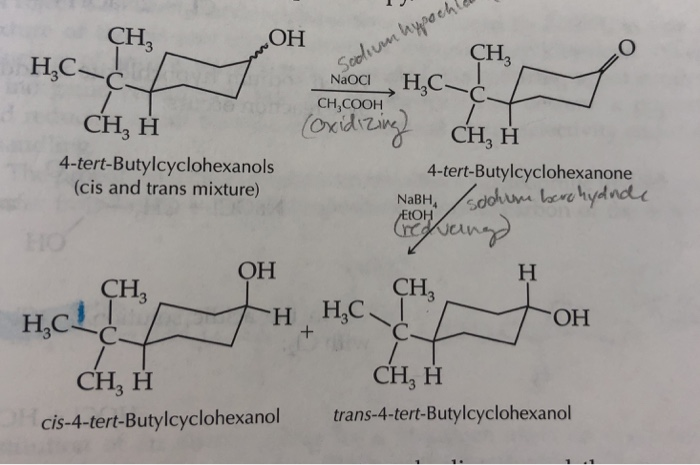
The purpose of this experiment is to reduce ketones 4-tert-Butylcyclohexanone to. This is a 3 week project that will evaluate the stereochemistry of the oxidation-reduction interconversion of 4-tert-butylcyclohexanol and 4-tert-butylcyclohexanone. CH 3 он CH CHCOOH CH H 4-tert.
In the reduction of 4-tert-butylcyclohexanone you obtain 92 trans-4-tert-butylcyclohexanone. The purpose of this experiment was to complete a reduction reaction. Dissolve 0 g of 4-tert-butylcyclohexanone in 6 mL of 95.
CSUN Chemistry and Biochemistry department 1st Semester Organic Chemistry LabStereoselective Reduction of 4-tert-butylcyclohexanoneRefChem 333 lab manual sp. Solid sodium borohydride is added to the flask containing 4-tert-Butylcyclohexanone. Week 1 you will oxidize the commercially available 4-tert-butylcyclohexanol which is.

Reduction Of 4 Tert Butylcyclohexanone Felkin Anh Youtube
Reduction Of 4 T Butylcyclohexanone

Exp 7a Sodium Borohydride Reduction Of 4 Tert Butylcyclohexanone Flashcards Quizlet
No comments for "Reduction of 4 Tert Butylcyclohexanone Mechanism"
Post a Comment